COHESION
NUIG
The aim of COHESION is to develop a Core Outcome Set for use in clinical trials, and other studies, for interventions for the treatment of Neonatal Encephalopathy.
Developing a Core Outcome Set for use in clinical trials, and other studies, for interventions for the treatment of Neonatal Encephalopathy
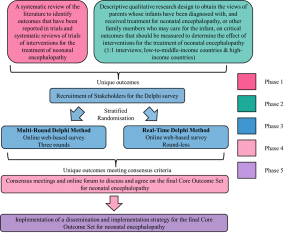
COHESION Study Phases
Phase 1: Systematic review of studies to identify outcomes measured in the treatment of neonatal encephalopathy. (STATUS: Completed)
Phase 2: Interviews with parents/ caregivers on outcomes they consider important in the treatment of neonatal encephalopathy. (STATUS: Completed)
Phase 3: An online Delphi survey to prioritise the importance of outcomes identified from Phase 1 and 2. (STATUS: Current stage)
Phase 4: An online consensus meeting to decide on the outcomes that will make up the final core outcome set. (STATUS: Pending)
Phase 5: Dissemination of the final core outcome set. (STATUS: Pending)
Supervisors
- Professor Declan Devane, Health Research Board-Trial Methodology Network (HRB-TMRN)/School of Nursing and Midwifery, National University of Ireland Galway
- Professor Eleanor Molloy, Chair of Paediatrics & Child Health, Trinity College Dublin
- Dr Patricia Healy, Research Fellow, National University of Ireland Galway
Description
One of the difficulties often faced by systematic reviewers when trying to synthesise the evidence from studies on a particular topic is heterogeneity in the outcomes measured in those studies. This means that reviewers are frequently unable to compare and contrast the findings of all, or even most, of the studies and a meta-analysis of the findings of all included studies is rarely possible. One of the suggested ways to address this problem, is to develop and apply agreed standardised sets of outcomes, known as ‘core outcome sets’ (COSs). The idea is that a COS should represent the minimum to be measured and reported in all trials, and other studies, on a specific condition, while accepting that if outcomes outside of the COS are also important in the context of the individual study they should be measured for that study. This use of the COS as a minimum across an entire research area would allow for the results of trials and other studies to be effectively compared, contrasted and combined, as appropriate.
The COHESION project will develop a Core Outcome Set for use in clinical trials, and other studies, for interventions for the treatment of Neonatal Encephalopathy.
NEPTuNE Scholar: Fiona Quirke

Fiona Quirke
Fiona is a PhD student with the HRB-TMRN based in the school of Nursing and Midwifery in NUI Galway. She has completed a BSc. in Biomedical science, in which she achieved a first class honours and graduated top of her class and has also completed a level 8 Diploma in Irish language literacy from NUI Galway. Fiona’s background in Biomedical Sciences has led her to pursue a number of research projects in areas of chronic pain, Parkinson’s disease and colorectal cancer. Her final year research project on Parkinson’s disease etiology was awarded an esteemed award from the Dean of Science in NUI Galway. In addition, she was awarded funding as a Summer Research Scholar from the College of Medicine, Nursing and Health Sciences, NUI Galway in 2017 to complete a research project investigating biomarkers in colorectal cancer. Her underlying interest in neuropharmacology and neurophysiology led her to pursue her HRB NEPTuNE project ‘COHESION: a core outcome set for the prevention and treatment of hypoxic ischaemic encephalopathy’. This project which is housed within the HRB Trials Methodology Research Network involves developing and applying an agreed standardised set of outcomes, known as ‘core outcome sets’ (COS) which represent the minimum to be measured and reported in all clinical trials on a specific condition. The project will also invite women and their partners as users of maternity services, midwives, obstetricians, paediatricians/neonatologists, researchers and groups with expertise in HIE prevention and treatment to provide their attitudes and experiences to ensure the best possible outcomes are decided upon.